A restorative treatment for mechanical CLBP
The information on this page is intended for Physicians.
ReActiv8® is an effective and durable treatment for your patients with mechanical Chronic Low Back Pain (CLBP) and multifidus muscle dysfunction.
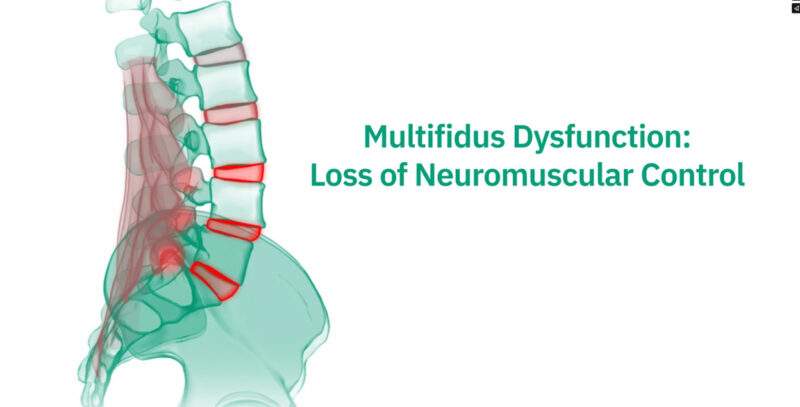
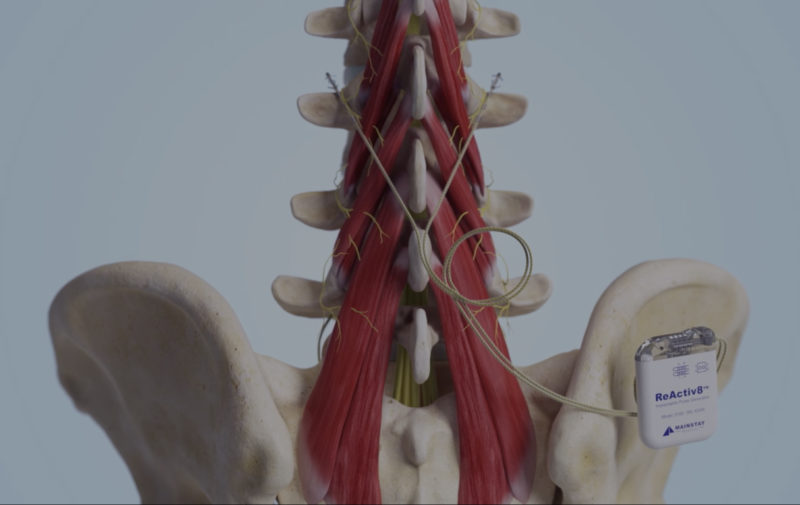
ReActiv8 Restorative Neurostimulation is a restorative therapy designed to address impaired neuromuscular control and degeneration of the multifidus muscle linked to mechanical chronic low back pain (CLBP). The ReActiv8 system overrides underlying multifidus inhibition by eliciting episodic, isolated contractions which, over time, can facilitate recovery from mechanical CLBP. This therapy can provide an effective treatment solution for your patients with mechanical CLBP.
ReActiv8, the first and only restorative device of its kind, uses a restorative treatment approach that targets underlying multifidus muscle dysfunction. Analgesic (or palliative) treatment options have limited effectiveness and durability in patients with mechanical CLBP. [1]
1 – Jensen MP, Brownstone RM. Mechanisms of spinal cord stimulation for the treatment of pain: Still in the dark after 50 years. European Journal of Pain (United Kingdom) 2019;23:652–9.
The ReActiv8 therapy targets the multifidus muscle with electrical pulses delivered through proprietary self-anchoring lead technology placed adjacent to the medial branch of the dorsal ramus. Post procedure, your patient conducts their own at-home therapy twice daily for 30 minutes per session.

The ReActiv8 System is Now Full Body MRI Conditional
Compelling Patient Outcomes
The ReActiv8-B Clinical Trial is an international, multi-center, randomized, active-sham controlled blinded trial comparing the ReActiv8 therapy Treatment Group to an active sham Control Group.
Long-Term Treatment Benefits
- ReActiv8® provides an effective, durable, and safe treatment option for carefully
selected patients with intractable CLBP and multifidus muscle dysfunction. - Trajectory and durability of clinical benefits are consistent with restoration of
neuromuscular control and muscle rehabilitation. - Restorative neurostimulation does not appear to be susceptible to loss of
efficacy. (i.e., decreasing effectiveness over time) - The safety profile of the therapy remained favorable compared to available
implantable neurostimulators for the treatment of other types of back pain. - An increasing proportion of participants are eliminating or decreasing opioid
consumption.
Gilligan C., Volschenk W., Russo M., et al (2024) Five-Year Longitudinal Follow-up of Restorative Neurostimulation Shows Durability of Effectiveness in Patients With Refractory Chronic Low Back Pain Associated With Multifidus Muscle Dysfunction. Neuromodulation, Article in Press, doi.org/10.1016/j.neurom.2024.01.006
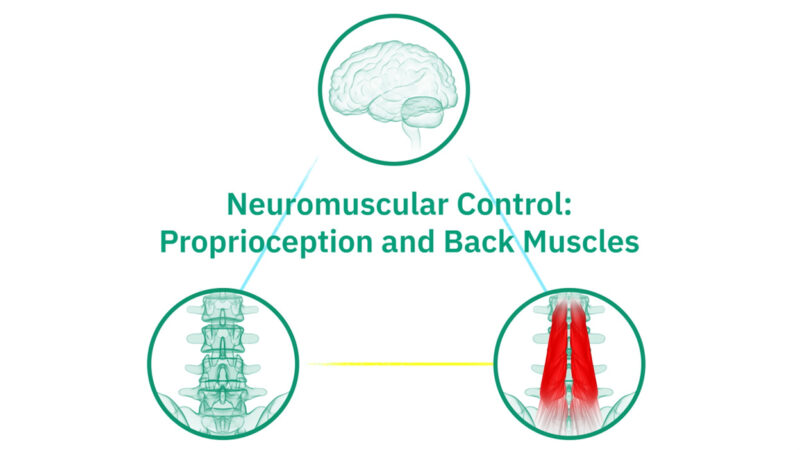
Neuromuscular Control: Proprioception and Back Muscles
Mechanical chronic low back pain results from an injury or stress on the tissues surrounding the spine, including soft tissues, muscles, bones, and joints. Often times, this type of pain is due to impaired neuromuscular control and neural inhibition of the multifidus, which is the largest stabilizing muscle in your back.
See the patients’ stories firsthand
ReActiv8 implanters and their clinical staff have seen patients’ lives change after treatment with ReActiv8. Watch their stories below below about their experience with mechanical CLBP.
The following videos contain stories from three of our international patients who experienced remarkable results from the ReActiv8 system. Patients’ stories are theirs only and not indicative of the results for all patients. Patient outcomes will vary. See paragraph below for US indication and product information.
Frequently Asked Questions
Reactiv8 is a treatment for adults with intractable mechanical chronic low back pain associated with multifidus muscle dysfunction, as evidenced by imaging or physiological testing. Candidates for the therapy have failed interventional treatment options including pain medications and physical therapy and are not indicated for spine surgery.
On average, people with CLBP who were treated with ReActiv8 Restorative Neurostimulation therapy had substantial reductions in pain, and improvements in disability and quality of life after one year. Given the restorative nature of the therapy, it may take some time for the pain relief and improvements in function to be experienced by the patient.
To assist in securing coverage from health insurance providers, we offer the ReActiv8 Support and Verification Program (R.S.V.P.) by partnering with experts specialized in working with health insurance companies and our physician partners. To learn more about enrollment for your clinic, contact us.
Take the first step for ReActiv8 Therapy Access
Contact us to learn how ReActiv8 can fit into your practice.